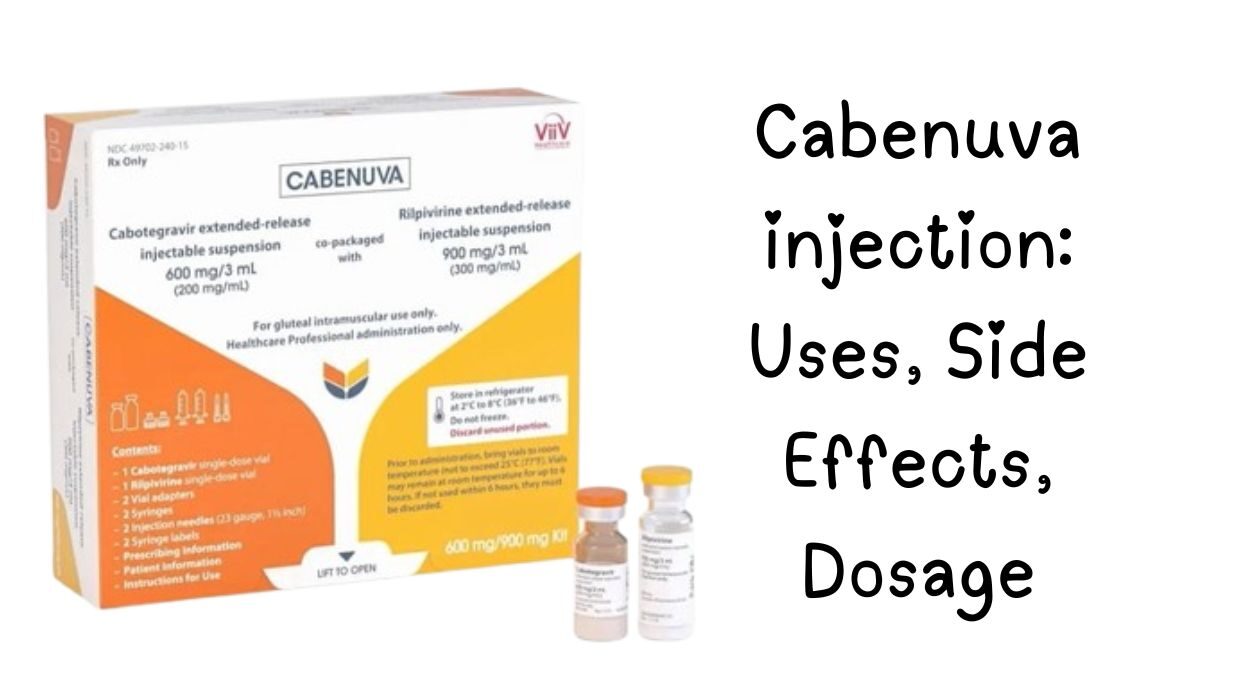
What is Cabenuva?
Cabenuva is the first complete long-acting HIV treatment regimen, combining two medications in a single monthly injection:
- Cabotegravir (integrase inhibitor)
- Rilpivirine (NNRTI)
FDA Approved For: HIV-1 infection in adults who are virologically suppressed (HIV-1 RNA <50 copies/mL)
Table of Contents
Cabenuva Uses & Benefits
Primary Use
✔ Maintenance therapy for HIV in stable patients
✔ Alternative to daily oral medications
Key Advantages
- Monthly injections (vs daily pills)
- Improved adherence (fewer missed doses)
- Discreet treatment option
Who Can Use It?
✅ Adults with undetectable viral load
✅ Those stable on current regimen ≥6 months
✅ Patients tired of daily pills
Cabenuva Side Effects
Most Common (≥2%)
| Side Effect | Frequency | Management |
|---|---|---|
| Injection site reactions | 80% | Warm compresses, rotation |
| Headache | 15% | OTC pain relievers |
| Fever | 10% | Monitor, hydrate |
| Fatigue | 8% | Rest, adjust activity |
Serious Risks
- Hypersensitivity reactions (rash, fever, fatigue)
- Liver issues (monitor LFTs)
- Depressive disorders (worsening mood)
Important Warnings
⚠ Not for initial HIV therapy – must be virologically suppressed first
⚠ Must complete oral lead-in (1 month of tablet form)
⚠ Potential resistance if doses are missed
⚠ Pregnancy risk – use effective contraception
Cabenuva Dosage
Standard Regimen
- Oral Lead-In (28 days):
- Cabotegravir 30mg + Rilpivirine 25mg (once daily)
- Monthly Injections:
- Cabotegravir 600mg + Rilpivirine 900mg (gluteal IM)
Missed Dose Protocol
- ≤7 days late: Get injection ASAP
- 7 days late: Restart oral therapy
Drug Interactions Table
| Drug Class | Example | Effect | Recommendation |
|---|---|---|---|
| Antacids | Tums | ↓ Rilpivirine absorption | Separate by 4+ hours |
| PPIs | Omeprazole | ↓ Efficacy | Avoid |
| Rifamycins | Rifampin | ↓ Levels | Contraindicated |
| Anticonvulsants | Carbamazepine | ↓ Levels | Use alternative |
Cabenuva Generic Name & Composition
Cabenuva contains two active drugs:
- Cabotegravir (integrase strand transfer inhibitor)
- Rilpivirine (non-nucleoside reverse transcriptase inhibitor)
Combination Type: Fixed-dose long-acting injectable suspension
Cabenuva Manufacturer Details
| Attribute | Information |
|---|---|
| Brand Owner | ViiV Healthcare |
| Parent Companies | GSK (78.3%), Pfizer (11.7%), Shionogi (10%) |
| Headquarters | Brentford, UK |
| FDA Approval Date | January 21, 2021 |
| Latest Innovation | Every-2-Month dosing (approved 2022) |
Manufacturing Facilities: Primarily produced in the UK and US with global distribution networks
Cabenuva Injection Price
United States Pricing
| Package | Average Price | Coverage |
|---|---|---|
| Starter Pack (1-month oral + 1 injection) | $5,400 | Most insurance/PBMs |
| Monthly Maintenance | $4,200/injection | Prior authorization usually required |
| 2-Month Dose (new option) | $7,800 | Increasing coverage |
International Pricing
- Canada: CA$2,900/month
- UK: £1,800/month (NHS covered)
- Australia: AUD 3,100 (PBS subsidized)
- India: Not yet available (expected 2025)
Patient Assistance: ViiV’s “Positive Action” program offers copay cards reducing cost to $0-50/month for eligible US patients
Cabenuva for PrEP (HIV Prevention)
While currently approved only for HIV treatment, studies show promise for prevention:
Current Status
- Apretude (cabotegravir alone): FDA-approved for PrEP (2021)
- Cabenuva: Being studied for:
- Dual-protection PrEP
- Event-driven prevention
- High-risk group protection
Potential Advantages Over Oral PrEP
✔ Better adherence (no daily pills)
✔ More consistent protection
✔ Discreet administration
Clinical Trial Results
| Study | Protection Rate | Population |
|---|---|---|
| HPTN 083 | 89% more effective than Truvada | MSM/trans women |
| HPTN 084 | 79% more effective | Cisgender women |