Atomic Absorption Spectroscopy (AAS) is a powerful analytical technique used to detect trace metals in environmental, clinical, and industrial samples with parts-per-billion (ppb) sensitivity. This guide covers how AAS works, its applications, advantages, and comparisons with other techniques—helping researchers, lab technicians, and students master this essential tool.
Table of Contents
🔍 What is Atomic Absorption Spectroscopy (AAS)?
AAS is a quantitative analytical method that measures elemental concentrations by analyzing how free atoms absorb light at specific wavelengths.
✅ Key Principle:
- Based on the Beer-Lambert Law (absorbance ∝ concentration).
- Each element absorbs unique wavelengths of light.
✅ Invented by: Alan Walsh (1950s).
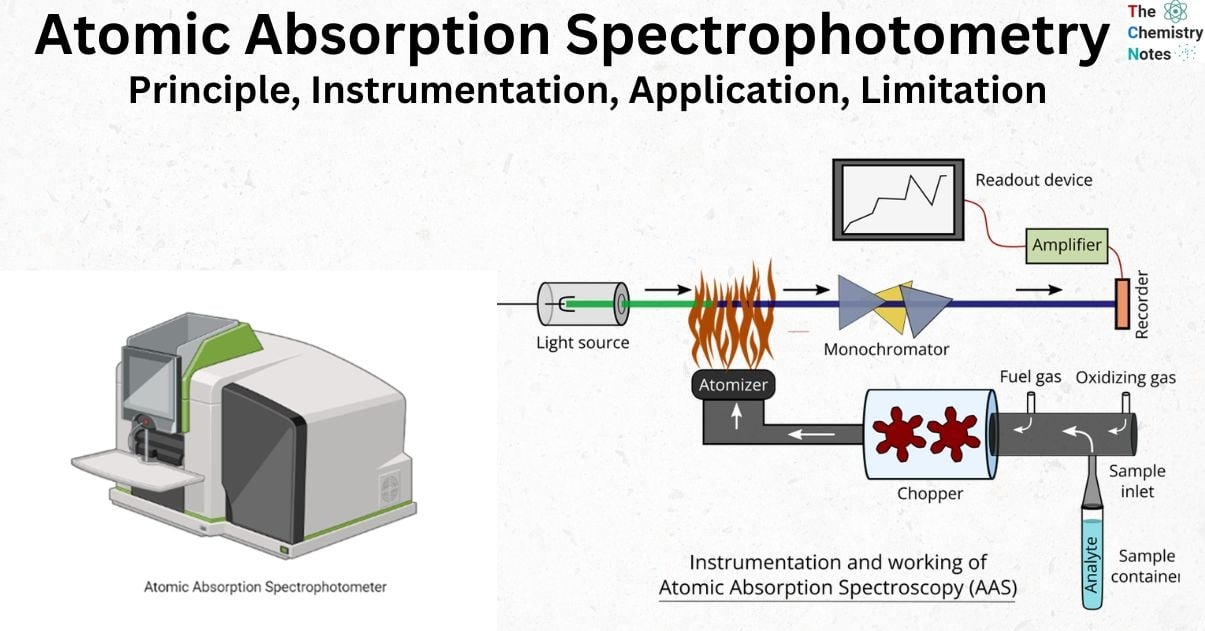
⚙️ How Does AAS Work?
1️⃣ Light Source (Hollow Cathode Lamp)
- Emits element-specific wavelengths.
2️⃣ Atomization (Flame or Graphite Furnace)
- Flame AAS (FAAS): Uses air-acetylene flame (fast, less sensitive).
- Graphite Furnace AAS (GFAAS): Electrically heated (ultra-sensitive, smaller samples).
3️⃣ Monochromator & Detector
- Isolates and measures absorbed light.
4️⃣ Readout System
- Displays concentration via calibration curves.
📊 Types of AAS Compared
| Type | Sensitivity | Sample Size | Best For |
|---|---|---|---|
| Flame AAS (FAAS) | Moderate (ppm) | Larger volumes | Routine metal analysis |
| Graphite Furnace AAS (GFAAS) | High (ppb) | Micro-samples | Trace metal detection |
| Cold Vapor AAS | Ultra-high (Hg only) | Small volumes | Mercury analysis |
| Hydride Generation AAS | High (As, Se, Sb) | Small volumes | Arsenic, selenium |
🔬 Top Applications of AAS
✔ Environmental Testing (heavy metals in water, soil, air)
✔ Food & Beverage Safety (lead, cadmium, arsenic detection)
✔ Clinical Diagnostics (blood, urine metal analysis)
✔ Pharmaceutical QC (elemental impurities in drugs)
✔ Forensics (toxicology, poisoning cases)
✅ Advantages of AAS
✔ High sensitivity (detects ppb levels)
✔ Cost-effective vs. ICP-MS
✔ Element-specific (minimal interference)
✔ Widely used & standardized
⚠️ Limitations of AAS
✖ Only detects metals/metalloids (not non-metals like C, N, O)
✖ Single-element analysis (slower than ICP-OES for multi-element)
✖ Matrix interference risks (requires sample prep)
AAS vs. Other Techniques
| Technique | Detection Range | Multi-Element? | Cost |
|---|---|---|---|
| AAS | ppb-ppm | ❌ No | $$ |
| ICP-OES | ppb-ppm | ✅ Yes | $$$ |
| ICP-MS | ppt-ppb | ✅ Yes | $$$$ |
| UV-Vis | ppm | ❌ No | $ |
🚀 Latest Advancements in AAS
🔹 Automated sampling (robotic arms, AI-driven analysis)
🔹 Portable AAS devices (field testing for mining, environmental work)
🔹 Hybrid techniques (AAS + chromatography for better accuracy)
🧰 AAS Maintenance & Calibration Tips
✔ Calibrate regularly (before each batch)
✔ Check nebulizer & gas flow (prevents clogging)
✔ Replace hollow cathode lamps (when intensity drops)
✔ Use matrix modifiers (reduces interference)
❓ FAQs About AAS
1. What elements can AAS detect?
Metals (Pb, Hg, Cd, Zn, Cu) & metalloids (As, Se).
2. How accurate is AAS?
Very accurate (ppb detection for most metals).
3. Can AAS analyze non-metals?
No, only metals/metalloids.
4. Flame vs. Furnace AAS?
- Flame: Faster, less sensitive.
- Furnace: Slower, ultra-sensitive.
5. How often should I calibrate?
Before each analysis batch or after maintenance.