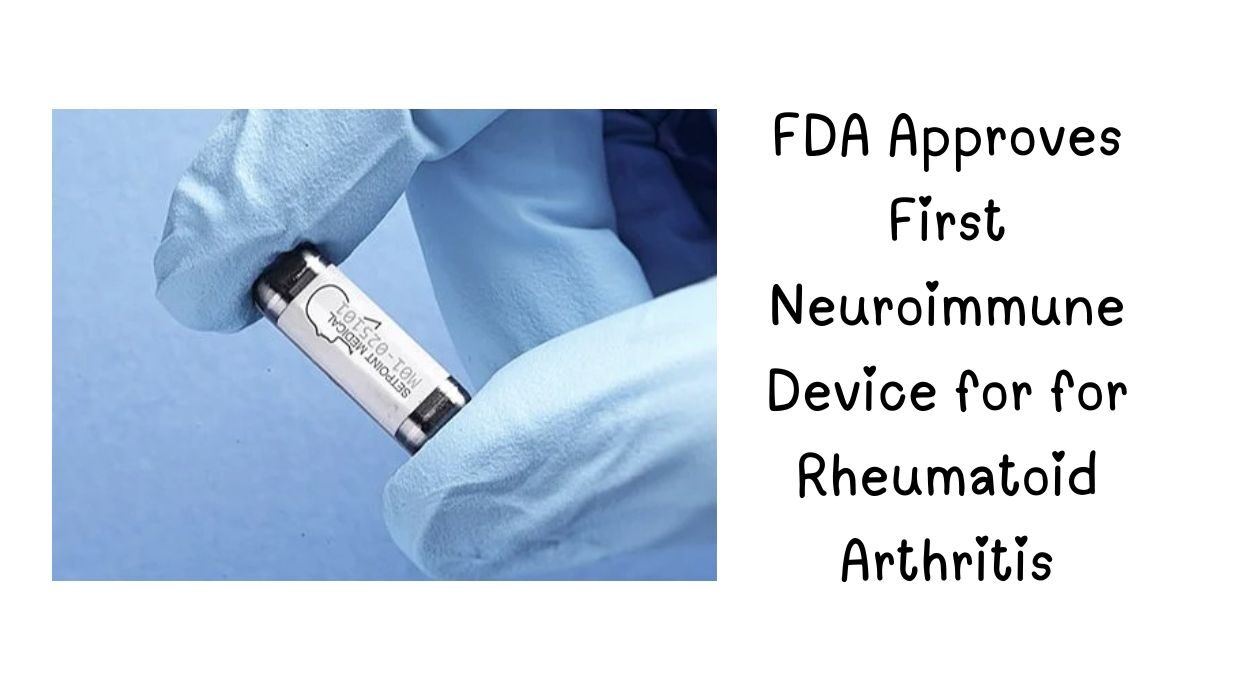
The U.S. Food and Drug Administration (FDA) has granted approval for the SetPoint System, a revolutionary neuroimmune modulation device designed to treat moderate-to-severe rheumatoid arthritis (RA) in adults who do not respond adequately to traditional therapies.
Key Highlights of the SetPoint System Approval
First-of-its-kind implantable device targeting RA via vagus nerve stimulation
Non-drug alternative for patients unresponsive to biologics & DMARDs
Clinically proven in the RESET-RA study with 75% of patients off biologics at 12 months
Minimally invasive outpatient procedure with 10-year battery life
Table of Contents
What is the SetPoint System?
The SetPoint System is an implantable neurostimulation device that delivers daily electrical pulses to the vagus nerve, activating the body’s natural anti-inflammatory response. This innovative approach offers a drug-free alternative for RA patients struggling with biologic side effects or treatment resistance.
How Does It Work?
- Targets the vagus nerve to regulate immune response
- Reduces inflammation by stimulating the cholinergic anti-inflammatory pathway
- Automated therapy (once daily) for long-term disease management
RESET-RA Study
The FDA approval was based on the RESET-RA trial, involving 242 patients with moderate-to-severe RA. Key findings:
| Outcome | Results |
|---|---|
| ACR20 Response (3 months) | Significantly higher vs. sham device |
| Disease Activity Reduction | Sustained improvements at 12 months |
| Freedom from Biologics | 75% of patients off biologics at 1 year |
| Safety Profile | 1.7% serious adverse events (well-tolerated) |
🔹 Expert Insight:
“This approval marks a turning point in autoimmune treatment—using the body’s neural pathways to fight inflammation.”
— Dr. Mark Richardson, Harvard University (Principal Investigator, RESET-RA Study)
Who is Eligible for the SetPoint Device?
The SetPoint System is approved for RA patients who:
✔ Do not respond to biologic DMARDs (e.g., Humira, Enbrel)
✔ Cannot tolerate side effects of current RA medications
✔ Seek a long-term, drug-free solution
FAQs About the SetPoint Neuroimmune Device
1. How is the SetPoint device implanted?
- Minimally invasive outpatient procedure (under local anesthesia)
- Small implant placed near the vagus nerve in the neck
2. How often does it deliver therapy?
- Once daily automatic stimulation (programmable settings)
3. How long does the device last?
- Up to 10 years before battery replacement is needed
4. What are the Neuroimmune Device side effects?
- Low risk (1.7% serious adverse events in trials)
- Possible mild hoarseness or cough (temporary)
5. Will insurance cover the SetPoint System?
- Coverage discussions ongoing—check with providers for updates
Why This Approval Matters for RA Patients
- New hope for treatment-resistant rheumatoid arthritis
- Avoids long-term drug dependency (biologics, steroids)
- Potential to slow disease progression naturally