For the first time, patients with macular telangiectasia type 2 (MacTel) have an FDA-approved treatment that can slow vision loss. The ENCELTO implant (revakinagene taroretcel-lwey) delivers continuous neuroprotection to preserve retinal cells, offering new hope for this rare, blinding eye disease.
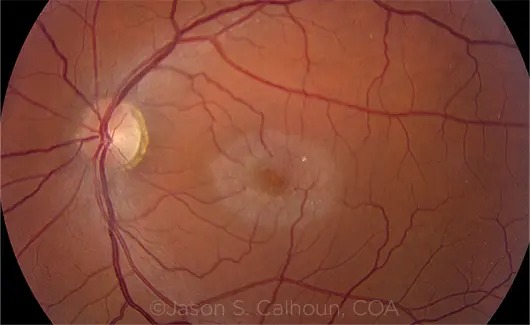
What Is MacTel?
Macular telangiectasia type 2 (MacTel) is a rare, progressive retinal disease that destroys central vision—critical for reading, driving, and recognizing faces. Until now, no treatments existed to stop its progression.
Table of Contents
How Does the ENCELTO Implant Work?
The ENCELTO implant is a revolutionary, cell-based neuroprotective therapy. It’s not a temporary fix; it’s designed for long-term, sustained delivery of a crucial therapeutic protein directly to the retina.
- The Key Ingredient: The implant delivers a protein called ciliary neurotrophic factor (CNTF), a naturally occurring molecule known to protect nerve cells. In the eye, CNTF acts as a powerful neuroprotectant, shielding vulnerable retinal neurons from damage and delaying the degenerative process.
- The Technology: The device consists of genetically modified retinal pigment epithelial (RPE) cells, which are housed within a tiny, collagen-based capsule. RPE cells naturally help nourish the retina, and in this case, they are modified to continuously produce and release CNTF.
- Surgical Precision: The capsule is surgically implanted in the back of the eye. This strategic placement ensures that the CNTF is delivered directly to the targe
ENCELTO Clinical Trial Results
Two Phase III trials (228 patients, 47 global sites) confirmed:
| Outcome | Trial 1 | Trial 2 | Combined Results |
|---|---|---|---|
| Reduction in retinal cell loss | 54.8% | 30.6% | Significant slowing |
| Microperimetry (vision sensitivity) | Improved | Mixed | Positive trend |
| Reading speed | No change | Slight improvement | Needs more study |
🔹 Key Finding: ENCELTO preserves retinal structure better than sham treatment.
Who Can Get ENCELTO?
✅ Diagnosed with MacTel
✅ Early to moderate stages (before severe vision loss)
✅ Failed other treatments (if applicable)
Is ENCELTO a Cure?
No—but it slows disease progression, helping patients retain vision longer.
Side Effects & Safety
Common Side Effects
- Mild eye discomfort post-surgery
- Temporary inflammation
Serious Risks (Rare)
- Infection at implant site
- Retinal detachment (extremely rare)
Overall, ENCELTO is well-tolerated with minimal complications.
Cost & Insurance Coverage
- Expected to be expensive (exact pricing pending)
- Insurance coverage may vary (check with provider)
- Patient assistance programs likely available
FAQs About ENCELTO
1. How long does ENCELTO last?
The implant is designed for long-term, continuous CNTF release—likely years.
2. Is ENCELTO covered by Medicare/Medicaid?
Pending approval, but specialty drug coverage is expected.
3. Can ENCELTO restore lost vision?
No, but it slows further degeneration, preserving remaining vision.
4. What’s the success rate?
54.8% reduction in retinal cell loss in one trial, 30.6% in another.
5. Are there alternatives to ENCELTO?
Currently, no other FDA-approved MaccTel treatments exist.